Jun 01, 2025
503B Compounding Pharmacy
503B Compounding Pharmacy: Cleanroom Requirements, Compliance, and Modular Solutions
Introduction: What Is a 503B Compounding Pharmacy?
A 503B compounding pharmacy is a specialized pharmaceutical facility registered with the U.S. Food and Drug Administration (FDA) as an outsourcing facility. Unlike traditional 503A pharmacies that compound medications based on individual patient prescriptions, 503B pharmacies are authorized to manufacture large batches of sterile drugs without patient-specific prescriptions—provided they meet strict regulatory and quality standards. These facilities serve hospitals, surgical centers, and healthcare providers with ready-to-use, sterile medications intended to streamline care, reduce preparation errors, and improve patient safety.
Established under the Drug Quality and Security Act (DQSA) of 2013, the 503B designation created a new regulatory category for compounders that operate at the intersection of pharmacy and manufacturing. To ensure safety at scale, 503B pharmacies must follow current Good Manufacturing Practices (cGMP), undergo FDA inspections, and operate in facilities that mirror pharmaceutical manufacturing plants in terms of environmental control, documentation, and validation.
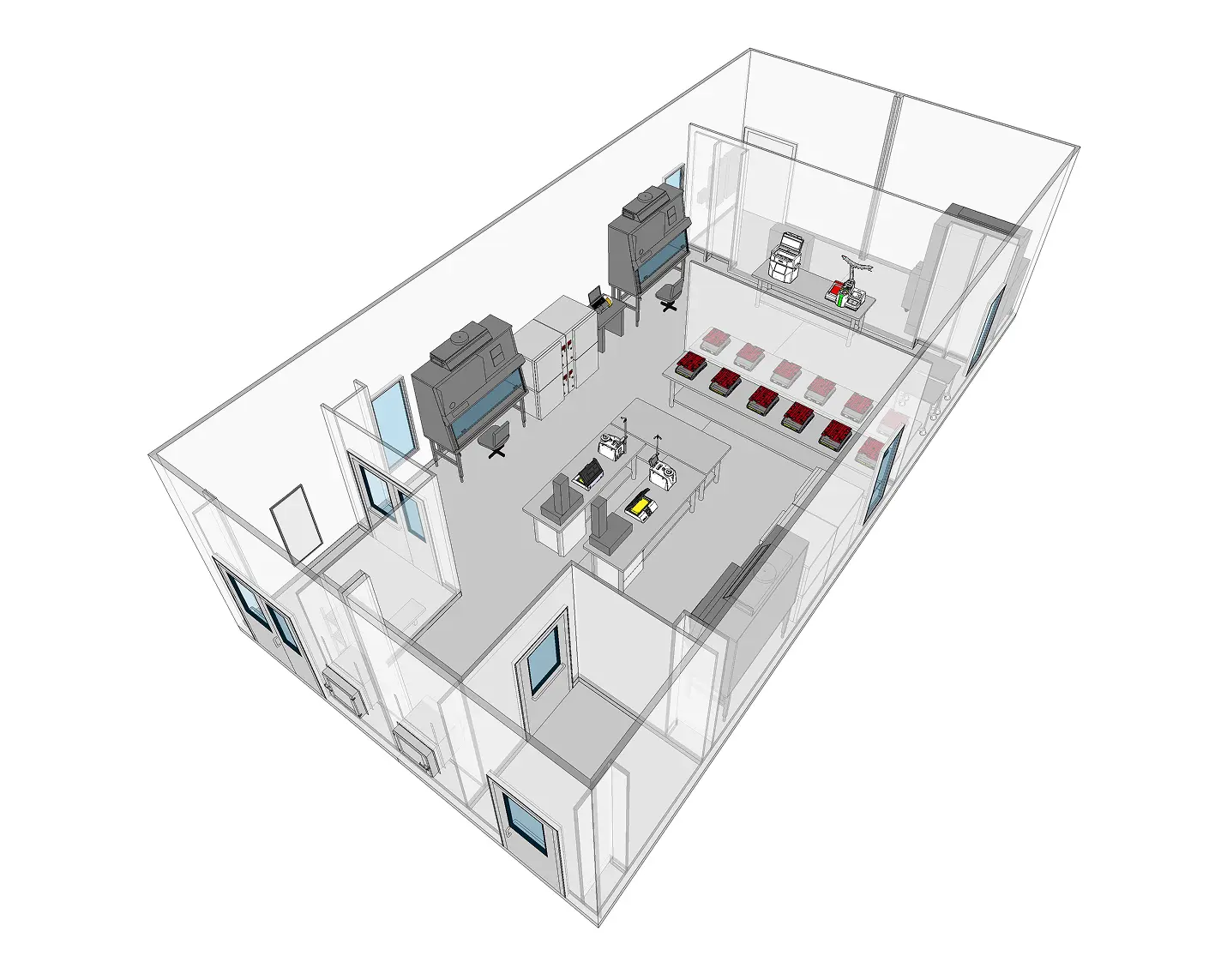
At the heart of every 503B outsourcing facility is a robust cleanroom environment. ISO-classified cleanrooms are required for sterile drug production, and the design, operation, and monitoring of these spaces are scrutinized by regulators. This article explores the unique role of 503B pharmacies, the cleanroom standards they must meet, and how modular cleanroom PODs can accelerate compliance and production readiness for sterile compounding operations.
503A vs. 503B: Regulatory Differences
The distinction between 503A and 503B compounding pharmacies lies primarily in their scope of practice, level of regulatory oversight, and applicable quality standards. Understanding these differences is essential for healthcare providers, compounding professionals, and facility planners designing cleanrooms that align with each category.
503A Pharmacies operate under Section 503A of the Federal Food, Drug, and Cosmetic Act (FDCA). These facilities compound medications based on valid, patient-specific prescriptions and are regulated primarily by state boards of pharmacy. While they must follow United States Pharmacopeia (USP) chapters such as <797> and <800> for sterile and hazardous drug compounding, they are exempt from the FDA’s full cGMP requirements. Inspections are usually conducted by state regulators, and batch sizes tend to be small and customized.
503B Outsourcing Facilities, established under Section 503B of the FDCA, are held to significantly higher standards. They may produce large batches of sterile compounded drugs for hospitals, clinics, and other healthcare institutions without requiring individual prescriptions. However, this exemption comes with heightened responsibility: 503B facilities must register with the FDA, comply with full cGMP standards, and undergo routine FDA inspections. Unlike 503A pharmacies, they are subject to adverse event reporting, product listing, and GMP-compliant manufacturing practices akin to pharmaceutical production facilities.
Cleanroom design implications follow naturally from these regulatory differences. A 503A pharmacy may use ISO Class 5 laminar flow hoods in ISO 7 buffer rooms, with less stringent requirements for facility zoning and documentation. In contrast, a 503B outsourcing facility must operate in purpose-built cleanrooms that meet FDA expectations for air cleanliness, pressure differentials, gowning protocols, environmental monitoring, and process validation. These cleanrooms must be able to support media fills, stability testing, and batch traceability—tasks not typically required of 503A operations.
FDA Oversight and cGMP Requirements for 503B Pharmacies
503B outsourcing facilities are regulated as drug manufacturers under the FDA’s current Good Manufacturing Practice (cGMP) framework, outlined in 21 CFR Parts 210 and 211. This designation imposes rigorous facility, process, and documentation standards designed to ensure the safety, identity, strength, quality, and purity of compounded medications. For cleanroom environments, cGMP compliance shapes every aspect of design, construction, operation, and monitoring.
Key cGMP requirements that impact cleanroom infrastructure include:
- Environmental Control and Cleanroom Classification: Critical aseptic processing must take place in ISO Class 5 environments with ISO 7 or better background support areas. All cleanrooms must be designed to maintain specified particle and microbial levels, with validated airflow patterns and pressure differentials to prevent contamination.
- Validation and Qualification: Cleanrooms and all supporting systems must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These activities must be documented with predefined acceptance criteria and include routine requalification.
- Environmental Monitoring (EM): The facility must implement a robust EM program that includes particle counting, viable air sampling, surface sampling, and pressure monitoring. Alert and action limits must be established and tied to risk-based corrective actions.
- Cleaning and Disinfection: The cleanroom must support routine cleaning and disinfecting procedures that include sporicidal agents. All surfaces must be compatible with cleaning agents and resistant to microbial colonization.
- Gowning Procedures and Zoning: Proper gowning areas (ISO 7 or better) and airlocks are required to minimize personnel-related contamination. Pressure cascades and personnel flow paths must be designed to reduce cross-contamination.
- HEPA Filtration and HVAC Design: The HVAC system must support HEPA-filtered unidirectional airflow in ISO 5 areas and maintain appropriate air changes per hour (ACH) in background rooms. Recirculated air must be appropriately filtered, and filter integrity must be verified regularly.
- Documentation and Audit Readiness: All cleanroom operations—including cleaning, EM, maintenance, deviations, and CAPA—must be documented and ready for FDA inspection. Modular cleanroom systems with built-in monitoring and digital infrastructure can simplify audit readiness.
In summary, 503B facilities operate under manufacturer-level expectations, requiring cleanrooms that deliver not only particle and microbial control but also verifiable, repeatable, and documented performance. Cleanroom builders and facility planners must approach 503B projects with a GMP mindset, using validated systems and designs that stand up to FDA scrutiny.
Cleanroom Design Requirements for 503B Compliance
Designing a cleanroom for a 503B compounding pharmacy requires a comprehensive, GMP-aligned approach that integrates contamination control, operational efficiency, and regulatory readiness. Because these facilities are subject to FDA inspections and full cGMP requirements, the cleanroom must be designed not only to meet ISO standards but to support all documentation, monitoring, and validation protocols expected of a pharmaceutical manufacturing site.
Key cleanroom design elements for 503B compliance include:
- Zoning and Pressure Cascade Strategy: Cleanrooms must be zoned according to the criticality of the process, with ISO Class 5 aseptic areas protected by ISO 7 buffer zones and ISO 8 ante-rooms. Properly engineered pressure cascades ensure that cleaner zones maintain positive pressure relative to adjacent areas, minimizing contamination ingress. Interlocked doors, airlocks, and pass-through chambers support controlled movement of personnel and materials.
- Gowning Rooms and Workflow Optimization: Gowning must occur in ISO 7 or better environments using defined entry and exit paths to prevent cross-contamination. Unidirectional personnel flow, clear signage, and ergonomic room layouts help support SOP adherence and reduce operational risks.
- Wall, Ceiling, and Floor Materials: All surfaces must be smooth, non-shedding, resistant to disinfectants, and easy to clean. Modular panels made from coated aluminum or stainless steel are preferred for walls and ceilings, while seamless epoxy or vinyl is used for flooring with coved edges to prevent microbial buildup.
- HVAC and HEPA Filtration Systems: The HVAC system must deliver controlled temperature, humidity, and air changes per hour (ACH) consistent with ISO classification targets. ISO 5 zones often use 100% ceiling-mounted HEPA coverage with unidirectional airflow. Recirculated air must be filtered, and HEPA filter integrity must be tested during commissioning and at regular intervals.
- Integrated Environmental Monitoring: Environmental Monitoring Systems (EMS) should be built into the cleanroom from the outset. These systems continuously track nonviable and viable particle counts, pressure differentials, temperature, and humidity. Real-time alerts, data logging, and trend analysis are critical for GMP compliance.
- Utility Integration and Cleanability: All piping, lighting, and utilities should be flush-mounted or concealed to avoid particle traps. Cleanroom-grade lighting fixtures, sealed outlets, and hands-free controls reduce contamination risks and support daily cleaning operations.
- Validation Access and Testing Infrastructure: Builders must include test ports, sampling stations, and access points that simplify qualification and validation activities such as airflow visualization, pressure mapping, and microbial sampling.
A well-designed 503B cleanroom serves as a production asset that enhances efficiency, reduces downtime, and facilitates regulatory inspections. Modular cleanrooms accelerate this process by embedding many of these design elements into pre-engineered, factory-tested units ready for validation upon installation.
ISO Classification Requirements in 503B Facilities
Cleanroom classifications for 503B outsourcing facilities are based on ISO 14644-1 standards, which define air cleanliness by particle concentration. The classification levels required for a 503B facility depend on the specific operations taking place in each zone and are enforced through both USP and FDA cGMP guidance.
The standard ISO classification hierarchy for a compliant 503B cleanroom includes:
- ISO Class 5: This classification is required for aseptic processing areas where sterile drugs are exposed to the environment—such as during vial filling, syringe preparation, or sterile filtration. These zones are typically achieved using laminar airflow workstations (LAFWs), biosafety cabinets (BSCs), or isolators. ISO 5 conditions must be maintained continuously during operations and are subject to the most stringent monitoring requirements.
- ISO Class 7: ISO 7 areas serve as background environments for ISO 5 operations. These buffer zones must maintain appropriate pressure differentials and support unidirectional airflow strategies. They house PECs like LAFWs and are critical for gowning, staging sterile components, and compounding sterile drugs.
- ISO Class 8: Ante-rooms and material transfer areas often fall under ISO Class 8. These rooms support proper transitions into cleaner environments and are designed to control personnel and material ingress. ISO 8 rooms also support hand hygiene and preliminary gowning before entering buffer zones.
Specific environmental conditions associated with each ISO class include:
- Particle count limits: Defined for ≥0.5 µm particles, with ISO 5 allowing no more than 3,520 particles per m³, ISO 7 allowing 352,000, and ISO 8 allowing 3,520,000.
- Air changes per hour (ACH): ISO 5 typically requires 240–300 ACH, ISO 7 requires 30–60 ACH, and ISO 8 generally operates at 20 ACH or more.
- HEPA coverage: ISO 5 areas often require 100% ceiling HEPA coverage with laminar (unidirectional) airflow, while ISO 7 and 8 use mixed-flow systems with targeted filtration.
Meeting these ISO classifications requires more than correct airflow rates. It involves validated performance under operational conditions, proper training of personnel, documented cleaning protocols, and a responsive EM program that identifies and addresses deviations in real time. Builders and operators must align every design and procedural detail with the classification goals for each space.
Validation, Documentation, and FDA Inspection Readiness
For 503B outsourcing facilities, the ability to demonstrate consistent cleanroom performance through robust validation and documentation is critical to FDA approval and ongoing compliance. Prefabricated Modular cleanrooms simplify this process by embedding validation into the design, fabrication, and commissioning process—supporting faster inspection readiness and lower operational risk.
Factory Acceptance Testing (FAT)
Each modular POD undergoes comprehensive FAT prior to shipment. This includes particle count testing, pressure cascade verification, filter leak testing, airflow velocity mapping, and temperature/humidity control validation. Test results are documented and included in the cleanroom’s compliance package.
Installation and Operational Qualification (IQ/OQ)
Prefabricated Modular systems streamline on-site qualification with predefined protocols, checklists, and documentation. IQ confirms that the cleanroom has been installed according to specifications. OQ verifies that all systems perform within acceptable parameters under simulated operating conditions. Modular builders often provide support or turnkey services for these activities.
Performance Qualification (PQ) and Media Fill Support
PQ testing confirms that the cleanroom maintains ISO classification and GMP compliance under actual operating conditions. This includes environmental monitoring during aseptic processing and simulated media fill runs to verify sterility assurance. Modular cleanrooms are designed with the infrastructure to support PQ sampling and operator qualification.
Documentation Packages
Every modular cleanroom is delivered with an extensive documentation bundle, including:
- Material specifications and certifications
- HVAC design and airflow diagrams
- FAT reports and test protocols
- Maintenance schedules and SOP templates
- Cleaning and EM integration guides
- Change control procedures
These documents simplify the creation of site master files, validation master plans, and audit trail documentation—key elements during FDA inspections.
21 CFR Part 11 Compliance
Digital monitoring systems used in modular cleanrooms meet the requirements for electronic records and signatures under 21 CFR Part 11. This ensures that environmental data is secure, traceable, and audit-ready.
Inspection Readiness and Risk Mitigation
Modular cleanrooms minimize inspection risks by delivering standardized, validated environments that have already met rigorous internal quality checks. Facilities using modular PODs demonstrate a proactive approach to contamination control, process integrity, and GMP alignment—attributes that inspectors look for when assessing risk.
In a 503B setting, inspection readiness is not a one-time event—it is a continuous state. Cleanroom providers can help customers maintain readiness by offering preventative maintenance and other ongoing services.
Building 503B Facilities with Compliance at the Core
503B compounding pharmacies occupy a critical space in the healthcare supply chain—delivering high-quality, ready-to-use sterile medications that support hospitals, surgical centers, and providers nationwide. But operating at this scale means embracing the responsibilities of a pharmaceutical manufacturer. From air cleanliness and gowning protocols to documentation, environmental monitoring, and batch traceability, every aspect of the facility must be engineered for cGMP compliance and FDA scrutiny.
Cleanroom infrastructure is the backbone of any successful 503B operation. Poorly designed or inadequately validated cleanrooms can result in product recalls, warning letters, or shutdowns. In contrast, facilities that invest in high-performance cleanrooms—especially prefabricated systems that arrive pretested and compliance-ready—gain a competitive advantage in speed, flexibility, and regulatory alignment.
Prefabricated cleanrooms offer 503B operators a faster path to market entry, scalable growth, and repeatable quality. With, integrated EMS systems, and complete documentation, these systems are not just construction solutions—they are compliance accelerators.
Whether you’re establishing a new 503B facility, upgrading an existing one, or expanding capacity, the cleanroom strategy you choose will determine your long-term success. By aligning with cleanroom experts and designing with GMP principles from the ground up, you can build a 503B pharmacy that is cGMP-compliant and built to last for years to come.
Frequently Asked Questions About 503B Compounding Pharmacies
What is a 503B compounding pharmacy?
A 503B compounding pharmacy is an FDA-registered outsourcing facility that compounds sterile drugs in bulk without requiring patient-specific prescriptions. These facilities must comply with cGMP regulations and undergo FDA inspections.
What are the cleanroom requirements for 503B facilities?
503B facilities must operate ISO Class 5 primary engineering controls within ISO Class 7 cleanrooms and ISO Class 8 ante-rooms. All cleanrooms must meet cGMP standards for airflow, pressure differentials, environmental monitoring, gowning, and cleaning protocols.
How is 503B different from 503A?
503A pharmacies compound drugs for individual patients based on prescriptions and are regulated by state boards of pharmacy. 503B facilities can compound in bulk and must follow FDA cGMP requirements and pass federal inspections.
Can prefabricated cleanrooms be used in 503B pharmacies?
Yes. Prefabricated cleanrooms are increasingly used in 503B facilities due to their rapid deployment, validated performance, and integrated compliance features. They are ideal for new builds, expansions, or rapid response to other changes..
What role does the FDA play in 503B facility oversight?
The FDA inspects 503B outsourcing facilities for cGMP compliance, product safety, and process control. Facilities must be registered, list their compounded products, and submit to regular inspections and adverse event reporting.