Jun 01, 2025
Sterile Compounding
Sterile Compounding: Cleanroom Requirements, USP Compliance, and the Role of Modular Facilities
Introduction: What Is Sterile Compounding and Why Does It Matter?
Sterile compounding is the preparation of injectable medications, infusions, ophthalmic solutions, and other sterile drug products in a controlled environment designed to prevent microbial contamination, pyrogens, and particulate matter. Unlike standard drug dispensing, sterile compounding involves combining, diluting, repackaging, or manipulating drug substances in ways that require precise environmental control, expert handling, and strict adherence to regulatory standards.
Used in hospitals, surgical centers, infusion clinics, and pharmacies, sterile compounded products often bypass the body’s natural defense systems. As a result, even minimal contamination can lead to serious infections, adverse reactions, or patient harm. For this reason, sterile compounding is one of the most tightly regulated areas in pharmacy practice and pharmaceutical manufacturing.
The importance of sterile compounding has only grown with increased demand for customized medications, shortages of commercially available products, and the need for ready-to-use, unit-dose preparations that improve workflow in healthcare settings. However, the risks are high—and the regulatory burden is significant. Facilities engaged in sterile compounding must operate within ISO-classified cleanrooms, follow detailed procedural controls, and comply with standards like USP <797> and <800> as well as cGMP if registered as 503B outsourcing facilities.
This article examines what sterile compounding involves, what cleanroom requirements apply, how USP standards shape facility design and operations, and how modular cleanrooms offer a modern solution for ensuring speed, safety, and compliance.
USP <797>: The Foundation of Sterile Compounding Standards
United States Pharmacopeia (USP) Chapter <797> provides the primary regulatory framework for sterile compounding in the United States. Enforceable by state boards of pharmacy and referenced by the FDA, USP <797> sets the standards for how compounded sterile preparations (CSPs) are prepared, handled, and stored. It outlines environmental requirements, personnel training, quality assurance measures, and facility design criteria—all aimed at minimizing the risk of contamination during sterile compounding.
Key requirements under USP <797> include:
- ISO-Classified Environments: All CSPs must be prepared in ISO Class 5 Primary Engineering Controls (PECs), such as laminar airflow workbenches (LAFWs), biological safety cabinets (BSCs), or compounding aseptic isolators (CAIs). These PECs must be located within a buffer room classified as ISO Class 7, with an ISO Class 8 ante-room providing personnel and material access.
- Personnel Training and Garbing: Staff involved in sterile compounding must undergo initial and ongoing training, pass media fill tests and gloved fingertip sampling, and follow rigorous garbing procedures using sterile gowns, gloves, face masks, and hair covers. Gowning must occur in the ante-room before entering the buffer area.
- Environmental Monitoring: USP <797> requires routine monitoring of viable and nonviable airborne particles, as well as surface sampling. Facilities must establish action and alert limits, investigate excursions, and document corrective actions.
- Cleaning and Disinfection Protocols: All cleanroom surfaces must be cleaned and disinfected according to a documented schedule using sterile cleaning agents, including a sporicidal disinfectant at least monthly.
- Beyond-Use Dating (BUD): USP <797> provides detailed guidance on assigning BUDs based on sterility risk levels, compounding methods, and whether sterility testing is performed.
- Facility Certification and Recertification: Cleanrooms and PECs must be certified every six months to confirm ISO classification and airflow integrity. Certification includes airflow velocity measurements, HEPA filter integrity testing, and smoke studies to verify unidirectional airflow.
USP <797> was updated in 2022 with a stronger emphasis on risk-based decision-making, clearer definitions of Category 1 and Category 2 CSPs, and increased flexibility for facilities using isolators. Whether a facility is a hospital pharmacy, specialty compounding center, or 503B outsourcing facility, USP <797> is the foundation for designing compliant sterile environments.
Types of Sterile Compounded Preparations and Risk Levels
Sterile compounding encompasses a wide range of preparations, each with different complexity, contamination risk, and regulatory requirements. Understanding these categories helps guide cleanroom classification, compounding procedures, and environmental monitoring protocols.
Category 1 CSPs
These are low-risk, short beyond-use date preparations intended to be used within 12 hours at room temperature or 24 hours if refrigerated. Category 1 CSPs must be prepared in an ISO 5 PEC located within a segregated compounding area (SCA) that is not necessarily ISO-classified but must meet stringent cleaning and operational controls.
Category 2 CSPs
These include all CSPs not classified as Category 1 and require more robust facility controls. They must be prepared in an ISO 5 PEC located within an ISO 7 buffer room, which itself must be supported by an ISO 8 ante-room. Category 2 CSPs can have extended BUDs and typically undergo sterility testing, depending on storage conditions and risk level.
Hazardous CSPs (HDs)
Sterile compounding of hazardous drugs, such as chemotherapy agents, must follow both USP <797> and <800>. These preparations require Containment Primary Engineering Controls (C-PECs), such as Class II BSCs, housed within Containment Secondary Engineering Controls (C-SECs), typically ISO 7 buffer rooms operating under negative pressure relative to adjacent areas.
Other Risk Factors
Risk classification can also be influenced by the number of manipulations, the complexity of the compounding process, whether sterility is preserved throughout the procedure, and the source of ingredients used (e.g., sterile vs. non-sterile components).
These distinctions directly impact cleanroom design and operational protocols. For example, a Category 2 facility preparing extended BUD CSPs must include HEPA-filtered air systems, airlocks, dedicated gowning areas, and enhanced environmental monitoring. A Category 1 SCA, while less complex, still requires stringent workflow control and routine surface disinfection.
As regulations evolve, many facilities are transitioning from open-air PECs to isolators and from SCAs to full ISO-classified cleanroom suites to expand capabilities and reduce regulatory risk.
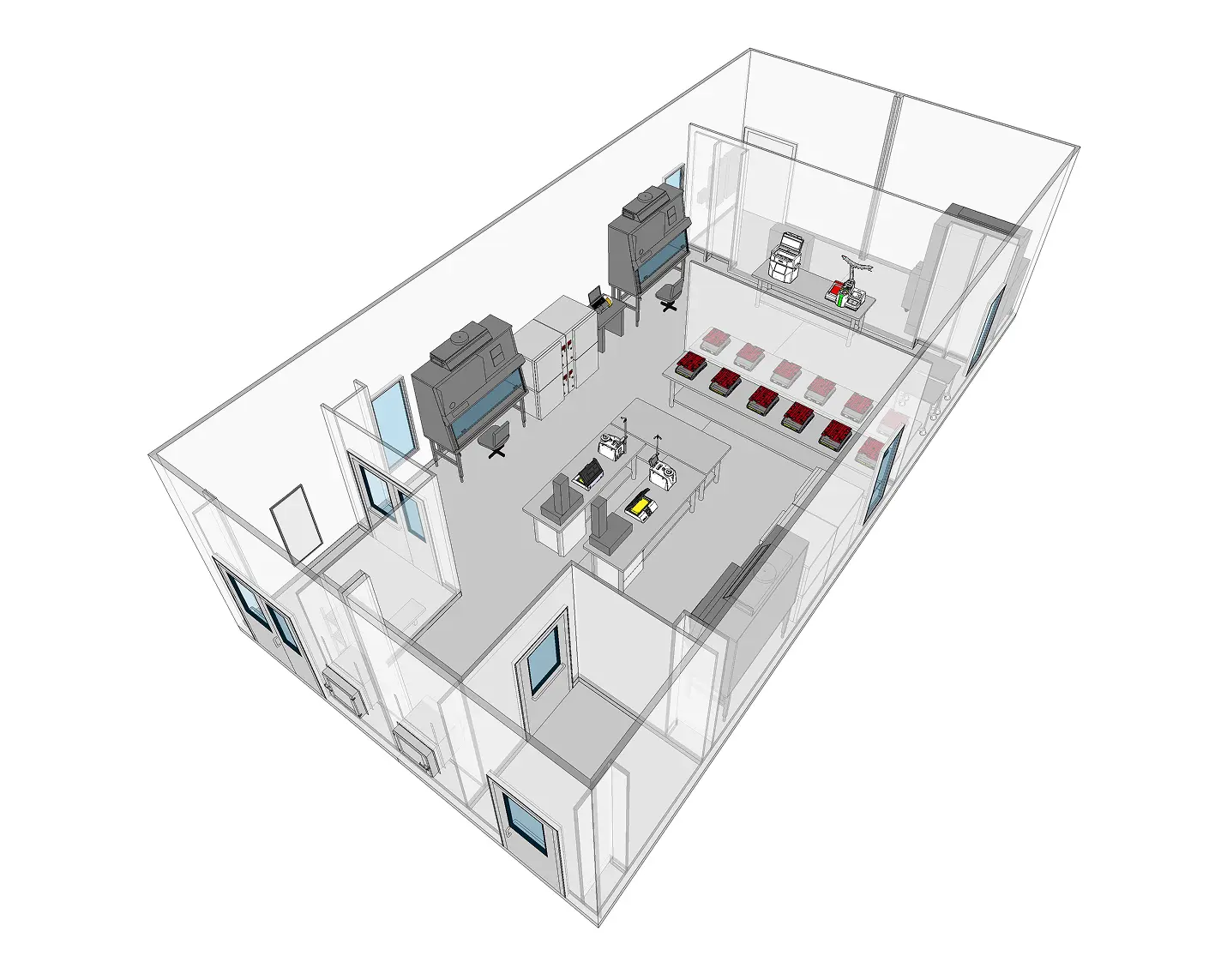
Cleanroom Requirements for Sterile Compounding Facilities
The core of any sterile compounding operation is the cleanroom environment. These controlled spaces are engineered to minimize particulate and microbial contamination during the preparation of compounded sterile preparations (CSPs). Whether in a hospital pharmacy, 503A compounding center, or 503B outsourcing facility, cleanroom requirements are defined by USP <797> and, where applicable, FDA cGMP standards.
Primary Engineering Controls (PECs)
All sterile compounding must occur within an ISO Class 5 PEC, such as a laminar airflow workbench (LAFW), biological safety cabinet (BSC), or compounding aseptic isolator (CAI). These devices use unidirectional HEPA-filtered airflow to protect the critical compounding zone from contamination. In most facilities, these PECs are placed within buffer rooms to provide additional environmental protection.
Buffer Rooms (ISO Class 7)
The buffer room housing PECs must be ISO Class 7 or better. It must maintain at least 30 air changes per hour (ACH), be supplied with HEPA-filtered air, and maintain positive pressure relative to adjacent areas (unless compounding hazardous drugs, which require negative pressure). Surfaces must be non-shedding, easily cleanable, and resistant to disinfectants.
Ante-Rooms (ISO Class 8)
An ISO 8 ante-room supports proper gowning, hand hygiene, and material staging before personnel or supplies enter the ISO 7 buffer room. The ante-room must maintain positive pressure relative to adjacent uncontrolled areas and support unidirectional personnel flow to reduce contamination risks. Dedicated gowning protocols and material transfer procedures are required to support environmental control.
Segregated Compounding Areas (SCAs)
For Category 1 CSPs, USP <797> allows the use of SCAs—unclassified areas where an ISO 5 PEC is placed in a designated room with specific cleaning and traffic flow controls. SCAs have limitations on BUD and are not acceptable for high-risk or hazardous compounding.
HEPA Filtration and Airflow Design
HEPA filters must be integrated into the ceiling plenum or PEC to maintain required ISO classifications. Airflow design must support laminar flow in critical zones and achieve necessary ACH for cleanroom classes. Pressure differentials between rooms must be continuously monitored and alarmed.
Room Layout and Material Flow
Cleanroom layouts must support unidirectional personnel and material flow to minimize cross-contamination. Gowning areas should be adjacent to ante-rooms, and pass-through chambers or airlocks are used for materials. Walls, floors, and ceilings must be seamless or properly coved to reduce particle buildup and microbial growth.
Construction Materials
Materials must be smooth, durable, and compatible with cleaning agents. Common choices include coated aluminum panels, stainless steel, epoxy flooring, and sealed light fixtures. Modular cleanrooms offer these features by default and are often favored for sterile compounding environments due to rapid deployment and high-quality finishes.
Cleanroom requirements vary based on the scope and complexity of compounding activities. Still, all facilities must design their cleanrooms to maintain control during both static and dynamic operations, support staff workflow, and meet the sterility expectations of regulators and patients alike.
Role of Environmental Monitoring and Quality Assurance
Environmental monitoring (EM) and quality assurance (QA) are essential pillars of any sterile compounding operation. A cleanroom alone does not guarantee sterility—only through rigorous, ongoing monitoring can a facility demonstrate that its compounding environment remains in control and compliant with USP <797> and, when applicable, cGMP expectations.
Nonviable Particle Monitoring
Continuous or routine monitoring of airborne particles ≥0.5 µm is required to verify ISO classification. In ISO 5 areas, real-time particle counters are often used during compounding to ensure no excursions occur. ISO 7 and 8 areas are typically monitored at defined intervals based on risk assessment.
Viable Air and Surface Sampling
Viable monitoring detects airborne or surface microbial contamination. Air samplers (e.g., impaction devices) are used in PECs and cleanroom spaces to collect and culture microorganisms. Surface sampling is performed using contact plates or swabs. Samples are incubated and analyzed to determine colony-forming units (CFUs), with action and alert limits set based on room classification.
Pressure Differential Monitoring
Differential pressure between cleanroom zones (e.g., buffer and ante-rooms) must be continuously monitored to confirm proper pressure cascades. Deviation from set limits must be investigated, corrected, and documented per facility SOPs.
Temperature and Humidity Control
Environmental conditions must be maintained within defined ranges to ensure operator comfort, product integrity, and equipment function. While not ISO-mandated, temperature (typically 20–22°C) and relative humidity (≤60%) are commonly targeted and tracked.
Trend Analysis and Corrective Action
EM results must be recorded, trended, and reviewed regularly. A spike in particle counts or microbial growth can indicate gowning failures, HVAC malfunctions, or cleaning gaps. QA teams must investigate these deviations, determine root causes, and implement corrective actions.
Media Fill Testing and Gloved Fingertip Sampling
As part of operator qualification and process validation, sterile compounding staff must pass media fill tests (simulated aseptic process runs) and gloved fingertip tests to confirm aseptic technique. These tests are repeated regularly and tied to ongoing EM data.
Documentation and Audit Readiness
All monitoring activities, SOPs, deviations, CAPAs, and QA reviews must be documented in a traceable manner. FDA inspections, accreditation surveys, and internal audits rely heavily on EM records to assess cleanroom control.
Effective EM and QA systems are not just regulatory checkboxes—they are the feedback loop that ensures the cleanroom operates as intended, operators follow aseptic technique, and CSPs are safe for patient use. Facilities that invest in robust QA infrastructure and automate EM through modular cleanroom systems are better prepared for inspections, product recalls, and evolving regulatory demands.
The Case for Modular Cleanrooms in Sterile Compounding
Modular cleanrooms are revolutionizing sterile compounding by providing turnkey, ISO-classified environments that are faster to deploy, easier to validate, and more adaptable to changing regulatory requirements. Whether for a hospital pharmacy, 503A facility, or 503B outsourcing operation, modular cleanrooms offer significant advantages over traditional construction in terms of speed, compliance, and flexibility.
Faster Time to Operation
Modular cleanrooms can be fabricated offsite while facility prep occurs in parallel, dramatically reducing total project timelines. Compared to stick-built cleanrooms—which may take 12 to 18 months—modular PODs can be delivered and validated in a fraction of that time, supporting urgent expansion or replacement needs.
Prevalidated ISO Classification
Each modular unit is built to meet ISO 5, 7, or 8 standards, with HEPA filtration, airflow design, and pressure differentials verified during factory acceptance testing. This reduces commissioning time and supports more efficient IQ/OQ/PQ processes.
Built-in Compliance Features
Modular cleanrooms are designed specifically for USP <797>/<800> and cGMP compliance, featuring smooth, cleanable surfaces; coved flooring; integrated EMS systems; and proper zoning for gowning, buffer, and ante-rooms. These features help meet both current and evolving regulatory expectations with minimal redesign.
Flexible Layouts and Scalability
As demand increases or workflows change, modular systems can be expanded or reconfigured. New units can be added to increase compounding capacity or separated to isolate hazardous drugs or accommodate different product types—all while maintaining compliance.
Improved Inspection Readiness
Leading modular providers deliver full documentation packages, including material certifications, airflow diagrams, FAT reports, and environmental monitoring integration guides. These documents support audit readiness and reduce the burden on internal QA teams.
Cost Predictability and Reduced Downtime
With defined scopes, standardized components, and minimal on-site construction, modular cleanrooms reduce the uncertainty and cost overruns common in custom builds. In facilities that cannot afford extended downtime, modular units can be installed and qualified quickly, minimizing production disruptions.
Modular cleanrooms offer sterile compounding facilities a smarter path to compliance—one that reduces risk, shortens time to market, and supports regulatory success through every stage of growth.
Building Safe, Compliant, and Scalable Sterile Compounding Facilities
Sterile compounding plays a vital role in modern healthcare, providing customized medications that support patient safety, workflow efficiency, and therapeutic flexibility. But the risks are high—requiring cleanroom environments that meet stringent ISO and USP standards and processes that are repeatable, documented, and inspection-ready.
Modular cleanrooms provide the infrastructure that today’s sterile compounding operations demand. Whether preparing Category 1 or Category 2 CSPs, handling hazardous drugs, or expanding into 503B-scale production, facilities that invest in modular systems gain the speed, quality, and compliance alignment needed to compete in a regulated marketplace.
By building with compliance at the core, embracing environmental control, and partnering with modular cleanroom experts, compounding facilities can ensure product safety, meet inspection demands, and position themselves for long-term success in the evolving pharmaceutical landscape.
Frequently Asked Questions About Sterile Compounding
What is sterile compounding?
Sterile compounding involves preparing injectable, ophthalmic, or infusion medications in a sterile environment to prevent microbial or particulate contamination.
What standards govern sterile compounding?
USP <797> is the primary standard for sterile compounding in the U.S. Facilities may also follow USP <800> for hazardous drugs and FDA cGMP guidelines if registered as 503B outsourcing facilities.
What are ISO cleanroom requirements for sterile compounding?
Sterile compounding requires ISO 5 conditions in the compounding zone, supported by ISO 7 buffer rooms and ISO 8 ante-rooms for Category 2 CSPs. Category 1 CSPs may be compounded in Segregated Compounding Areas (SCAs) under more limited conditions.
How often do cleanrooms need to be certified?
Cleanrooms and PECs must be certified every six months for ISO classification, airflow, HEPA filter integrity, and pressure differentials.
Are prefabricated cleanrooms suitable for sterile compounding?
Yes. Prefabricated cleanrooms offer pretested, cGMP-compliant environments designed to meet ISO 14644 and USP <797>/<800> standards. They reduce project timelines and simplify qualification.