Our readily deployable, integrated solutions and cleanroom infrastructures provide ease of scalability, full integration and fast turnaround across all process applications.
TOP APPLICATIONS
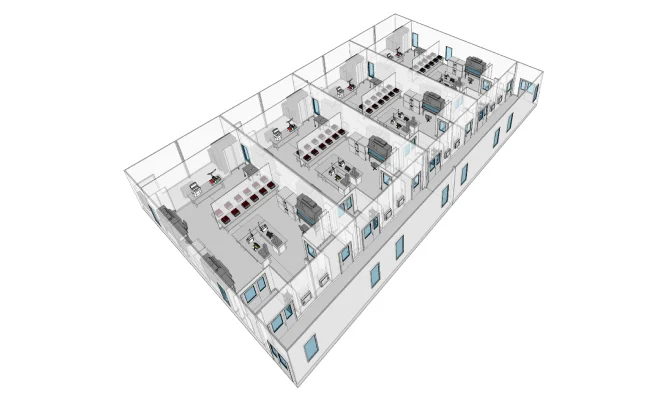
CELL THERAPY
Capacity scaling without interruption for the manufacturing of therapies to treat rare diseases.
Request Info
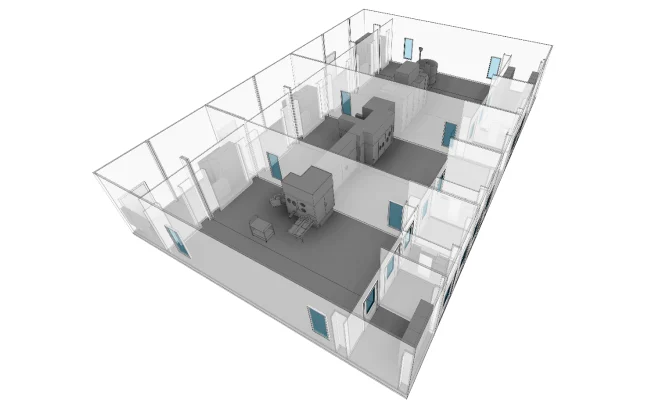
ASEPTIC FILLING
Fully integrated solutions for aseptic fill finish.
Request Info
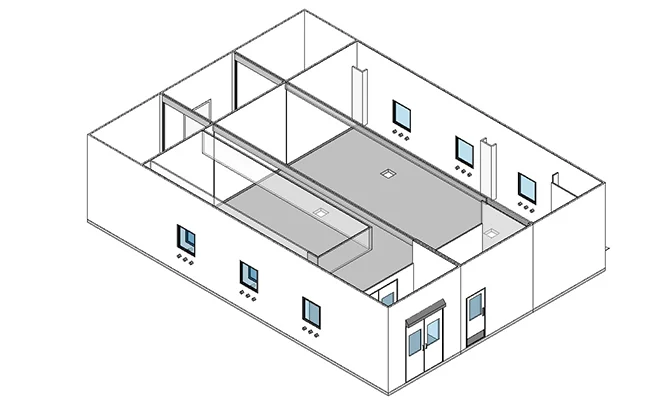
MONOCLONAL ANTIBODIES
Clinical to commercial scale solutions integrating both traditional and innovative process technologies
Request Info
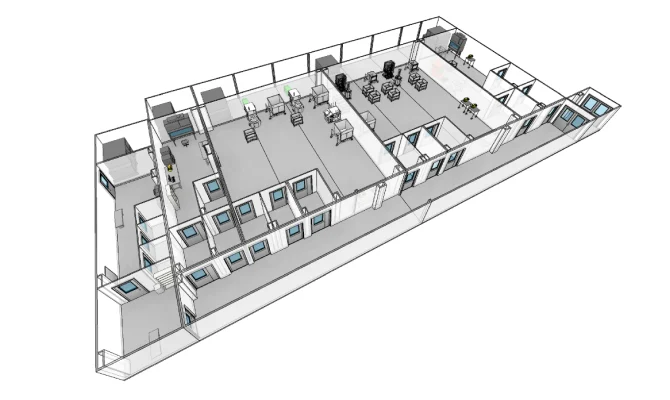
VIRAL VECTOR
Capacity scaling without interruption for the manufacturing of therapies to treat rare diseases.
Request Info
Life Sciences Applications
- Cell Therapy
- Gene Therapy/Viral Vector
- Aseptic Filling
- mAbs/Biologics
- Vaccines
- mRNA
- OSD
- ADCs
- Medical Device
- SUS Assembly
- Labs
Semiconductor
Applications
- R&D Suites
- Testing Labs
- Tooling Assembly
- Component Manufacturing











