Aug 25, 2025
Designing for Containment: Strategies for Safe and Compliant Cleanroom Facilities
Author: Pete Makowenskyj, G-CON, Sr. Director of Design Consulting
Patient safety is a topic often foremost in our minds when considering how to design and operate a pharmaceutical or biologics manufacturing facility. The FDA and EMA, two of the most influential global regulatory agencies in biopharmaceuticals, have various industry guidelines ensuring drug safety and compliance. Annex 1 within the EMA guidelines was recently revised, stressing the importance of Contamination Control Strategies (CCS) and Quality Risk Management (QRM) to ensure that microbial, particulate and endotoxin/pyrogen contamination is prevented in the final product.
While drug and patient safety are paramount, there are various drug modalities which pose a risk to operator safety during manufacturing. Antibody drug conjugates (ADCs), radiopharmaceuticals, and spore-forming microorganisms are just some of the types of agents that can pose a risk to operators in their facilities. While Annex 1 the FDA Guidance on Sterile Drug Products often cite drug and patient safety, there are only a few references in Annex 1 to containment needs or requirements. Rather, other organizations, such as the CDC, have been great sources of information when considering containment requirements and have published documents on biosafety and occupational exposure. in the case of spore-forming agents, the FDA did put out a guidance document which outlines containment strategies for such facilities.
When dealing with hazardous materials or biologics, it is critical that proper containment strategies are taken into account for safety of the operators and environment. While the focus of this white paper is on containment strategies, it is important to note that certain solvents or powders can also create concerns for explosion or fire ratings. In these instances, while the vapors or powders may not pose an immediate risk to operators upon exposure, when in the atmosphere at a critical concentration, they could pose an explosion risk. Design features need to be included to mitigate such occurrences.
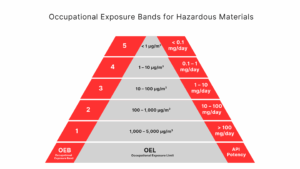
Figures 1 and 2 below, taken from the CDC papers cited above, outline the risks associated with biological agents and hazardous materials respectively. As the BSL or OEB number increases, so does the level of associated risk when those agents are present.
Figure 1: Biosafety level and associated risk
Figure 2: Occupational Exposure Bands for Hazardous Materials
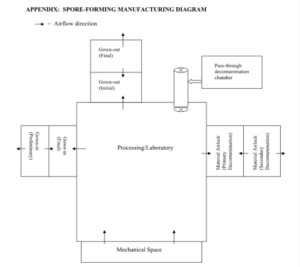
When designing a facility which deals with such hazardous materials/organisms, compliance and safety needs to be maintained. It can sometimes be the case that regulatory compliance and safety/containment practices may be contradictory with each other. A good example of this is when the hazardous component requires negative pressure to ensure proper containment, yet the activity should be done in a classified space which typically means the room should be at a higher pressure to ensure product safety. While many guidance documents do not provide explicit examples on how to handle this, the guidance on spore-forming agents does illustrate how the suites, airlocks and pressurization scheme should be set up to ensure compliance and containment.
Figure 3: Appendix from Guidance for Industry, Manufacturing Biological Intermediates and Biological Drug Substances Using Spore-Forming Microorganisms
The airlock and associated pressurization scheme are one of the critical focal points that need to be addressed to ensure proper containment. Depending on the level of risk, other items will need to be considered. These include:
- HVAC Zoning
- Recirculation vs Single Pass
- Bag in Bag Out Filter (BIBOs) or Return HEPAs
- Decontamination Strategy, i.e. VHP
- Egress Showers
- Room tightness and sealing of components
- Engineering vs Procedural Based Controls
- Unidirectional Flow Paths
- Single vs multimodal or multiproduct facility
As the risks per facility or project can vary, it is difficult to say one single design fits all the various design spaces that are needed. That is why it is critical to confirm not just the design space for today but also consider future facility output demands so that there is a certain level of future-proofing that can take place. Technologies such as pre-fabricated solutions often come into play to ensure a higher level of flexibility and future proofing. Additionally, pre-fabricated cleanrooms with integrated HVAC As the risks per facility or project can vary, it is difficult to say one single design fits all the various design spaces that are needed. That is why it is critical to confirm not just the design space for today but also consider future facility output demands so that there is a certain level of future-proofing that can take place. Technologies such as pre-fabricated solutions often come into play to ensure a higher level of flexibility and future proofing. Additionally, pre-fabricated cleanrooms with integrated HVAC.
About G-CON:
G-CON specializes in the design and construction of advanced cleanroom solutions for a wide range of industries. G-CON works closely with customers and A&Es to provide solutions that meet their specific needs, building PODular cleanrooms offsite, and providing modular and hybrid construction options. These offerings enable rapid deployment and easy configuration of cleanrooms while delivering the highest quality facilities, quickly and on time and on budget as well.
Contact Us:
For POD Cleanroom Inquiries, email sales@gconbio.com.