May 05, 2025
Optimizing cGMP Facility Design Space with a Hybrid Cleanroom Approach
Dennis Powers, Senior Vice President, Product & Strategy, G-CON Manufacturing
Combining on- and off-site construction approaches reduces project risk while offering benefits to schedule, flexibility, and cost of ownership for new cleanroom construction
INTRODUCTION
Modular and prefabricated construction approaches continue to gain recognition across multiple industries for the benefits they offer to the quality, timeliness, and flexibility of new manufacturing facilities compared to traditional stick-built construction. While some capital projects may benefit from a single approach, the optimal solution for most new facility construction projects is an integrated combination of off-site and on-site construction methods that enables project requirements to be met on schedule while providing the best value across the facility lifecycle. This hybrid approach is increasingly being adopted by biopharmaceutical manufacturers and contract development and manufacturing organizations (CDMOs) for new construction of current good manufacturing practice (cGMP) facilities. In this paper, we review industry drivers and risks for capital construction, with a focus on cleanroom infrastructure, and discuss the goals of using a hybrid approach while demonstrating its application and benefits using real-world examples.
DRIVERS OF COMPLEXITY AND RISK IN CAPITAL PROJECTS
Capital infrastructure projects in the biopharmaceutical manufacturing space are highly technical and incredibly complex. Regulations for cGMP biopharmaceutical product manufacturing [reviewed in (1)] require manufacturers to create control strategies for containment and segregation to prevent contamination of the product, operators, and outside environment. Many of these strategies involve engineering and facility controls that create appropriate personnel and material flows and ensure segregation by batch and/or modality. This includes facility designs with proper adjacency and directionality as well as highly complex, robust heating, ventilation, and air conditioning (HVAC) systems that consistently provide specified environmental conditions, airflows and pressure cascades (1). If a facility is used for sterile manufacturing, these processes require an extensive network of clean utilities which penetrate the cleanroom boundary. These penetrations and other architectural features within the cGMP footprint must be carefully considered when designing the cleanroom envelope. The cleanliness and robustness of the cleanroom is fundamental to meeting regulatory requirements and is dependent on architectural finishes; for example, some construction materials are less durable over time under cleaning regimens required in critical environments.
These complexities must be considered in the design of any facility; however, the first step is to identify the business case and mission-critical drivers, which form the project foundation. Schedule is typically the driver of utmost importance for capital projects, including target time to clinic, first to market, or response to listed drug shortages or a pandemic. Execution timeline and speed inform the engineering economics and often serve as a go/no-go for construction methodologies. Market authorization is a driver that is dependent on location, which heavily influences the design, project execution, and regulatory strategy. Utilization of a proposed facility for multiple modalities has cascading impacts on containment and segregation and greatly increases project complexity compared to single-modality manufacture. Throughput and scale are also very important drivers; however, a project team must consider not only short-term requirements but also future capacity increases in response to market changes. All process, business, and regulatory considerations need to be considered by the end user, consultants, solution providers, and other project team members in order to navigate the preceding drivers, which together impact the driver with the most influence on capital project approval – budget. A risk-sensitive approach to budgeting, which considers the level of certainty as appropriate for the design stage, is key.
With this in mind, project teams should evaluate the risks in parallel with the drivers during the early stages of project planning, since understanding these risks and their impacts will improve decision making and guide execution pathway selection. Important risks that should be considered during pre-project planning include delays and changes. Delays are inevitable in most capital projects and should be considered in light of how much tolerance the project and objectives have for such delay. For example, if delays to project schedule that impact timing of operational readiness result in delays to market or clinic, or in product stockout, clinical and commercial financial losses could be significant and are therefore inherently tied to the overall success of the project. Client-side changes should be considered as well. A greenfield construction project may take 3-5 years from planning to operational readiness. As projects evolve over this time period, additional clinical or marketing data may change the scope of the project or cause major process changes in throughput and scale. Understanding these risks and their probabilities is important in order to select a project execution approach that will mitigate these risks. If there is not a high level of project certainty, execution pathways that offer more flexibility may minimize cost increases and delays.
TRENDS IN PROJECT EXECUTION
Once critical project drivers are assessed and risk tolerances established, the project team can select the project execution approach. While some projects may benefit from a single construction approach, often the optimal solution lies in a combination of these methodologies within a hybrid approach. The design space should therefore be seen not as competing construction methodologies but as competing hybrid alternatives with varying combinations of off-site, prefabricated, and on-site methodologies. Hybridization generally allows projects to be executed faster, with a higher degree of certainty and cost. While ensuring the highest levels of quality, a hybrid approach can maintain flexibility during front end planning and identify solutions that allow facility scaling without interrupting on-site operations. Inclusion of modular and/or prefabricated construction in a hybrid approach can also alleviate strain on local resources, since suppliers often have dedicated installation teams that mobilize to the project location; this can be particularly important in emerging markets, where local resourcing of skilled labor with experience in critical environment construction may become an unforeseen bottleneck during project execution.
It is therefore important for a project team to understand the strengths of each of the three construction methodologies in order to leverage their benefits in appropriate areas of a new facility. Figure 1 provides a high-level qualitative assessment of the various factors that should be considered for each methodology. It is readily apparent that each approach has clear advantages. For example, traditional stick-built construction, which takes place at the project location using standard materials such as gypsum board and epoxy paint, has strengths in the area of history, experience, and flexibility; however, it rates lower in areas which confer risk, such as project length and schedule predictability (Figure 1, left). Conversely, prefabricated modules, where most of the infrastructure is constructed and tested at an off-site factory before being installed at the project site, rates highly for project duration, depreciation, and cost and schedule certainty, but has less history and the highest cost (Figure 1, right). Not surprisingly, modular construction, where architectural and envelope components are manufactured off-site and then assembled in the field, tends to rate between stick-built and prefabricated methods (Figure 1, center).
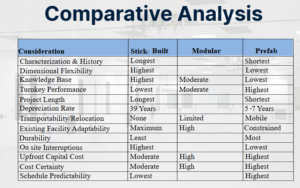
The impacts of these factors need to be weighted according to a project’s specific drivers and risks. However, even the above comparisons of stick-built vs. modular vs. prefabricated can be too rigid when evaluating new biopharmaceutical facility projects. Prefabricated modules may not be flexible enough for the entire space, modular panels may not be structurally supportable in a retrofit of an existing building, and stick-built may be neither fast nor robust enough to meet the project requirements. However, when these projects are viewed as a combination of various areas that each have different requirements, a hybrid solution can be tailored to meet specific project needs and mitigate risk.
AREA-BY-AREA ANALYSIS AS A KEY TO HYBRIDIZATION
It is important to identify the risk and criticality of each area within a facility to ensure the proper quality and containment controls are put in place. Quality tools such as Failure Mode and Effects Analysis (FMEA) enable quantitative scoring for each area of the manufacturing facility to determine its best construction approach. Generally, manufacturing suites and product-contacting areas score as the most critical, and areas like storage and corridors score as the least critical. For example, an inoculation suite is a highly classified area which provides the necessary environment for aseptic manipulations, requiring a higher degree of performance which would benefit from a modular or prefabricated approach, while an active pharmaceutical ingredient (API) release storage suite does not contain critical process equipment and would allow modular or even stick-built construction.
To further illustrate this approach, a generic cell therapy layout might contain process suites, cell therapy (CT) suites, entry locker space, quality control (QC) laboratories, and material handling areas (Figure 2). Traditionally, an individual cost estimate would be generated for each single-solution approach based on the project requirements; however, a hybrid approach applies the best methodology to the specific needs of different areas. One potential approach might therefore utilize prefabricated cleanrooms for the most critical areas, such as the CT and material handling suites, and use stick-built construction for areas such as the locker rooms and QC laboratories, where there are no manufacturing operations. An alternative hybrid approach might maintain the core CT suites as prefabricated cleanrooms but use modular cleanrooms for all logistics and QC areas, and use stick-built construction for the locker area, as shown in Figure 2. These represent only two of the possible hybrid approaches that could be used for this type of project; the optimal solution is the one that best addresses the project-specific drivers while also mitigating the business risks.
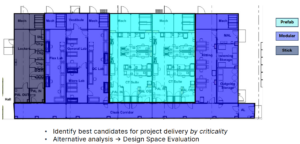
HYBRID CLEANROOM APPROACHES AND PROJECT SCHEDULE
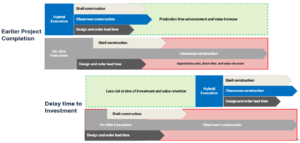
Project schedule is a critical element for every cleanroom project. For on-site stick-built or modular construction, project execution is sequential, requiring the shell building to be completed prior to cleanroom construction. Inclusion of prefabricated elements in a hybrid project approach enables projects to be completed faster through a parallel execution pathway (Figure 3, top), which allows prefabrication of cleanrooms off-site simultaneous with construction of the shell building. This enables manufacturers to complete capital projects faster and more efficiently, with reduced risk; thus, products reach the market faster, resulting in a higher rate of return on their investment.
Increased speed and efficiency using a hybrid cleanroom approach also offers the potential for delayed time to investment (Figure 3, bottom). The goal in this approach is to reduce the business risk by allowing more time to collect additional critical data, such as clinical trial results, further process characterization, or market condition data, before the start of construction. This facilitates a more efficient use of capital and resources with overlapping tasks, while also reducing risk.
EVALUATING THE COST OF HYBRID CLEANROOM APPROACHES
Historically, differing approaches to new cleanroom construction have been evaluated solely on upfront, direct capital cost, which provides incentive for bidders to exclude scope or diminish value in order to win the project contract. While cost per square foot can be a helpful benchmark, these numbers can be deceiving. It is more important to consider total cost of ownership, which not only includes direct cost but also accounts for indirect and operational expenses over the life of the asset. This requires transparency, collaboration, and knowledge transfer between end users, designers, and system providers to generate accurate estimations of net present value (NPV), internal return, and payback period. With hybrid cleanroom approaches, certain indirect costs can be reduced or even eliminated, leading to a reduced total cost of ownership relative to a traditional approach.
For example, we compared the total cost of ownership between a hybridized cleanroom project and a traditional on-site design for a recent 40,000 sq ft manufacturing facility project (Figure 4). Although the initial capital investment for the hybrid facility was slightly higher, we found that under identical project conditions at the same location, the hybrid project provided a more favorable economic outcome, with a higher NPV and a shorter payback period (Figure 4). There are two primary explanations for this finding. First, a hybrid solution enables faster time to operational readiness than traditional stick-built construction, which means both faster realization of revenue and an incremental increase in revenue generation. Additionally, modular or prefabricated cleanroom components may be depreciable over 7 years rather than the 39-year depreciation used for traditional commercial construction. Taken together, the data indicate that for this facility, a hybrid solution requires a slightly higher initial capital investment than a traditional stick-built approach, but would provide a better return on investment (ROI) (Figure 4).

To illustrate how the hybrid approach works in practice, we present three real-world case studies, below, each with unique goals and project drivers for which a hybrid approach was the optimal, successfully implemented solution.
Case studies 1-2: Expandability and Flexibility
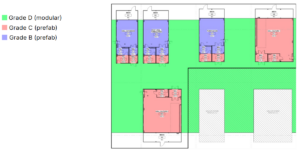
The key driver for the first biological drug manufacturing project was schedule, with an operational readiness target of 18 months from project start. On-site skilled labor needed to be minimized due to local labor scarcities, and flexibility needed to be incorporated into the design to allow future scale-out. A hybrid solution was designed where intensified core processes for production of monoclonal antibodies and other biologics at commercial scale (cGMP grades B and C) took place within prefabricated cleanrooms that were installed within a larger modular ballroom to facilitate movement of personnel and materials (cGMP grade D) between core process areas (Figure 5). This design allowed buffer and media totes to remain in the larger ballroom space, since all solution transfer was performed through connection ports. This design also allowed space for rapid scale-out and capacity increases through future installation of additional prefabricated cleanroom pods (Figure 5, lower right).
A second drug manufacturing project took a slightly different approach. The key driver for this facility was flexibility that would allow multimodal production as well as rapid changes in manufacturing capabilities with minimal downtime. This hybrid approach used prefabricated cleanroom pods connected directly to a modular, central ballroom (not shown). These pods contained preprogrammed unit operations that allowed rapid reconfiguration of the cleanroom suites in a bolt-on, “plug and play” fashion as needed for just in time (JIT) or on-demand manufacturing campaigns of different products. This arrangement also enabled future expansion through connection of additional prefabricated cleanroom pods to the ballroom while maintaining critical area containment. In addition to these benefits, this case study highlights the potential for prefabricated modules to be relocated and/or repurposed at the end of an initial project life cycle rather than decommissioned, which can extend the useful life and retain value of these assets.
Case study 3: Phased Construction for Planned Scale-Out
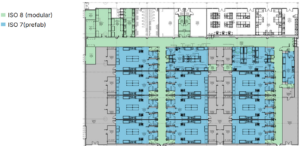
In this final case study involving construction of cell therapy manufacturing, the key driver was flexibility and planned scale-out, in this case by installing cleanrooms in multiple phases. This hybrid approach utilized prefabricated cleanroom pods for core critical process areas (Figure 6, blue) with modular cleanroom construction for connecting corridors and support areas (Figure 6, green). A single process suite composed of a bank of prefabricated pods was installed in phase one, while additional prefabricated clones of that suite were able to be installed in the building footprint in phases two and three to increase manufacturing scale and capacity without impacting current production.
CONCLUSION
Design and construction of biopharmaceutical manufacturing facilities are complex, requiring project design teams to clearly establish key drivers and risks early in the project planning stage. Optimization of cGMP design space requires assessment of multiple methodologies on a project-specific and area-by-area basis. A hybrid approach combines the strengths of stick-built, modular, and/or prefabricated construction methodologies and reduces risks in project execution, while meeting project quality, schedule, and performance requirements and maximizing return on investment.
REFERENCES
Covarrubias, C.E.; Rivera, T.A.; Soto, C.A.; Deeks, T.; Kalergis, A.M. Current GMP Standards for the Production of Vaccines and Antibodies: An Overview. Front Public Health. 2022, 10, 1021905. DOI: 10.3389/fpubh.2022.1021905
