
Prefab solutions
Cleanrooms for cell and gene therapy
providing advanced cleanrooms for cell
and gene therapy
G-CON specializes in providing advanced cleanrooms for cell and gene therapy (C>), addressing the unique challenges of advanced therapy medicinal products (ATMPs). Our prefabricated PODs® Cleanrooms are designed to meet the stringent requirements of cell and gene therapy manufacturing, offering flexibility, scalability, and rapid deployment to accelerate your path from research to commercialization.
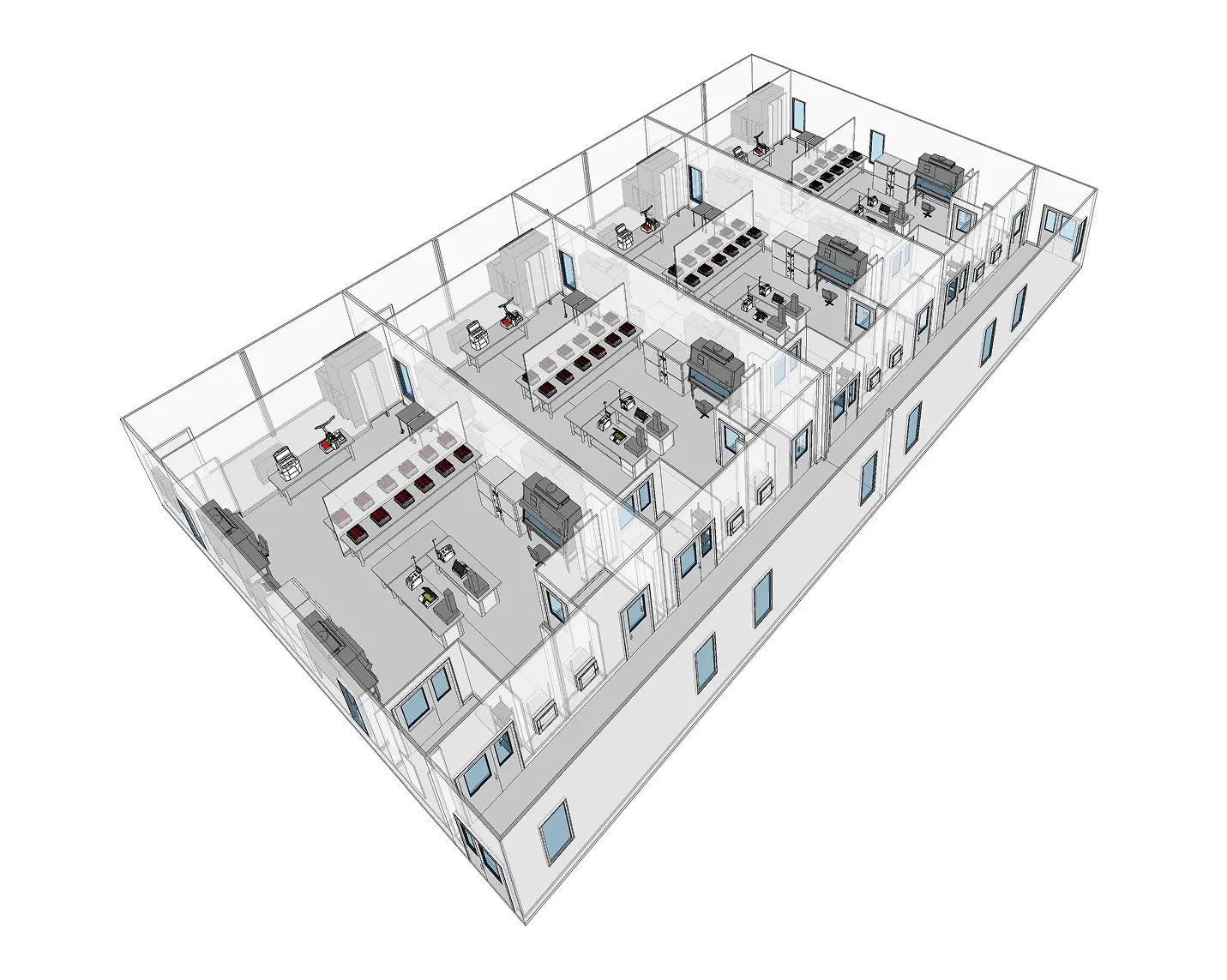
WHY CHOOSE G-CON POD® CLEANROOMS FOR CELL AND GENE THERAPY
G-CON is dedicated to advancing cleanroom solutions for cell and gene therapy through continuous innovation and adherence to the highest quality standards. Our cleanrooms
are designed to support the evolving needs of the cell and gene therapy industry, ensuring that our clients can bring life-changing therapies to patients efficiently and safely.
- Rapid Deployment: Our POD® Cleanrooms can be delivered and operational in as little as six months, significantly reducing time-to-market compared to traditional construction methods.
- Modular and Scalable: G-CON’s cleanrooms are modular, allowing for seamless scalability as your manufacturing needs evolve. This flexibility is crucial for accommodating the dynamic nature of cell and gene therapy production.
- Compliance and Quality: Our cleanrooms are built to comply with current Good Manufacturing Practices (cGMP) and are designed to meet the specific regulatory requirements of cell and gene therapy processes.
- Integrated Solutions: We offer fully integrated cleanroom solutions, including HVAC systems, environmental monitoring, and process utilities, ensuring a turnkey approach to your facility needs.
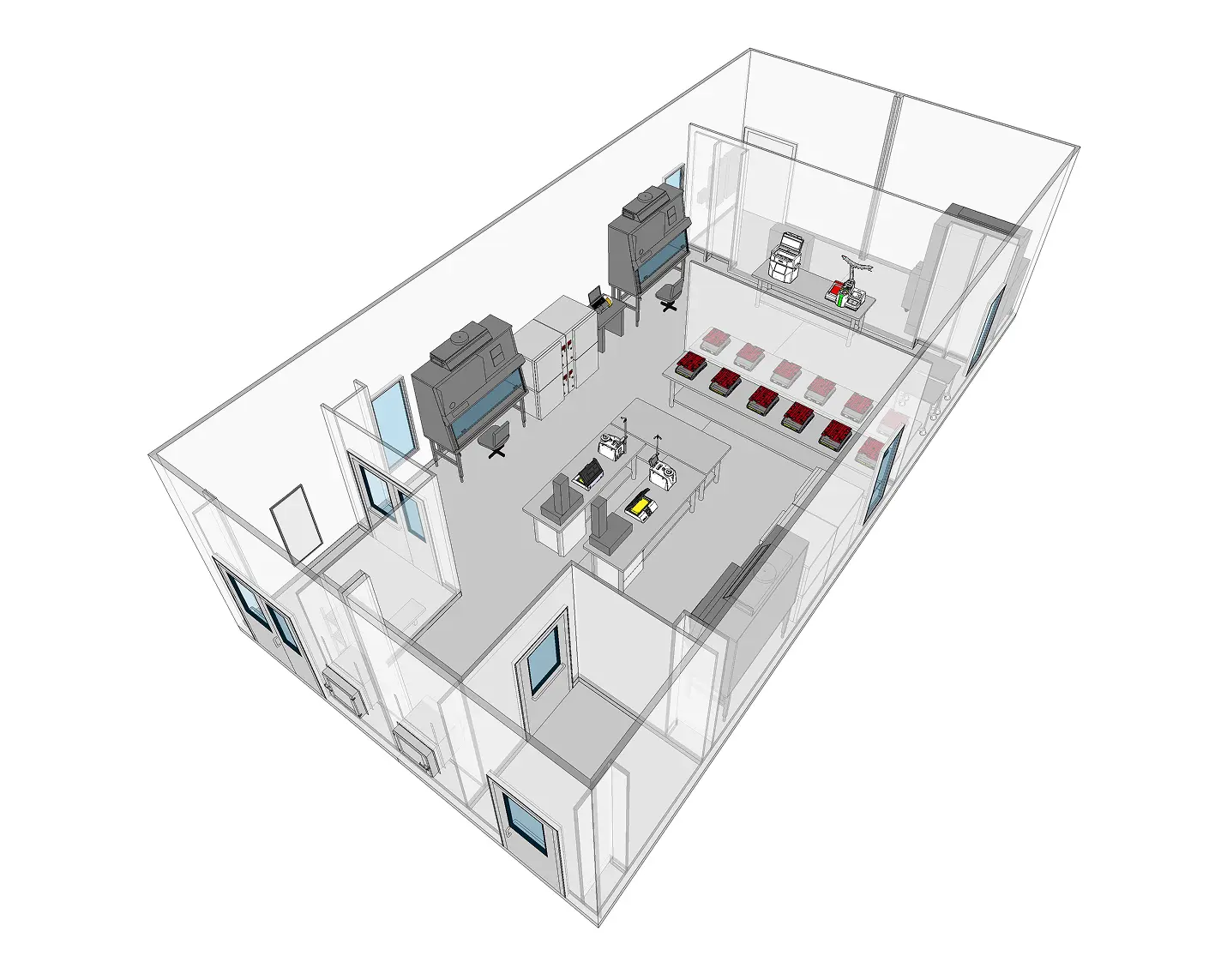
ADVANCED CLEANROOM FEATURES
POD® Cleanrooms for cell and gene therapy have an inherent design that is well suited for CG&T applications. POD® Cleanrooms are aligned with Biosafety Requirements associated with these modalities (BSL 2 or greater) and are equipped with cutting-edge features to support advanced therapy manufacturing.
- Integrated HVAC Systems: During the design phase, AHU zones are established to ensure proper segregation and containment within the process and still allow operational efficiencies to reduce energy costs. The integrated system is tested prior to leaving G-CON’s facility, and for critical applications can come with redundancies and failsafes in the case of mechanical or electrical failures.
- Containment: In addition to establishing compliant AHU zoning, POD® Cleanrooms are designed with appropriate transition areas, pressure schemes and when needed can be equipped with HEPA filtration on return chases or bag-in/bag out (BIBO) filtration on the exhaust.
- Validated Data Management System: POD® Cleanrooms can come with a built-in Environmental Monitoring System to ensure compliance (21 CFR Part 11 compliant) for data capture. While our integrated BMS runs the cleanrooms, the EMS system captures all relevant environmental conditions, such as temperatures, relative humidities, etc. Process equipment data and environmental monitoring data (viable and non-viable) can
also be sent to the EMS system.
APPLICATIONS OF POD CLEANROOMS IN CELL AND GENE THERAPY
Viral Vector Production
Providing controlled environments for the production of viral vectors such as lentivirus (LV) and adeno-associated virus (AAV), essential for gene therapy applications.
cell manufacturing
Facilitating the genetic modification manipulation and expansion of cells under sterile conditions, critical for cell therapy manufacturing.
Aseptic Fill Finish
Ensuring sterile conditions during the final formulation and packaging of cell and gene therapy products.